Abstract
OBJECTIVES: Currently there is no standard treatment option for AML patients not fit for intensive chemotherapy. Further clinical challenges include poorer prognosis associated with advanced age. AML treatments among this population have not been directly compared in clinical trials. Furthermore, naïve comparisons of results across multiple trials are subject to bias related to not accounting for within-trial differences from control. Standard (unadjusted) ITC accounts for within-trial efficacy of treatment versus control, providing a valid and robust approach to compare effectiveness between trials. Additionally, simulated treatment comparison (STC) applies ITC methodology and adjusts for differences in population baseline characteristics. Previous ITC and STC of overall survival (OS) demonstrated superiority of combination glasdegib and low-dose ARA-C (GLAS+LDAC) over azacitidine (AZA) or decitabine (DEC). This present study applied ITC and STC of clinical response outcomes between GLAS+LDAC and AZA or DEC among older AML patients unfit for intensive chemotherapy.
METHODS: Clinical outcomes for complete remission (CR) and CR plus CR with incomplete blood count recovery (CR+CRi) were indirectly compared between Phase II trial (n=116) GLAS+LDAC individual patient data (IPD) and published Phase III AZA results (n=488), and then between GLAS+LDAC IPD and published Phase III DEC results (n=457). Based on the Decision Support Unit STC guidelines from the National Institute for Health and Care Excellence, GLAS+LDAC IPD regression (which modelled response) was separately adjusted for AZA and DEC patient baseline characteristics. Stepwise models adjusted for key patient covariates and full models adjusted for all patient covariates mutually available between trials including age, sex, having denovo AML or not, bone marrow blast percentage, Eastern Cooperative Oncology Group performance status, cytogenetic risk factors, and hemoglobin level. Between trials, CR and CR+CRi were indirectly compared by standard ITC and STC using risk ratios (RR) with 95% confidence intervals (CIs).
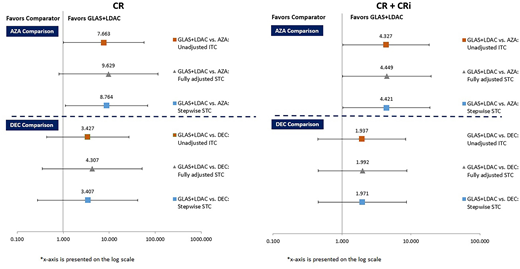
RESULTS: Naïve comparisons of CR [GLAS+LDAC (17.95%), AZA (19.50%), and DEC (15.70%)] and CR+CRi [GLAS+LDAC (24.36%), AZA (27.80%), and DEC (25.62%)] favored AZA. However, based on each of the three modelling techniques (standard ITC, STC full covariate adjustment, STC stepwise adjustment), GLAS+LDAC showed superiority over AZA.
Compared with AZA, standard ITC estimated that GLAS+LDAC patients were over seven and four times as likely to reach CR [RR=7.67 (1.02, 57.87)] and CR+CRi [RR=4.33 (1.02, 18.29)], respectively.
Using STC full covariate adjustment, GLAS+LDAC patients compared with AZA were over nine times as likely to reach CR [RR=9.63 (0.81, 113.95)] and over four times as likely to reach CR+CRi [RR=4.45 (1.01, 19.59)]. In the stepwise STC approach, GLAS+LDAC versus AZA similarly improved the likelihood of CR [RR=8.76 (1.13, 68.21)] and CR+CRi [RR=4.42 (1.04, 18.87)].
Comparing GLAS+LDAC to DEC, standard (unadjusted) ITC found numeric but not statistically significant improvement in CR [RR=3.43 (0.44, 27.00)] and CR+CRi [RR=1.94 (0.45, 8.43)]. STC methods for GLAS+LDAC versus DEC yielded trends similar to ITC. Non-significant trends in numeric favor of GLAS+LDAC were found using both full covariate adjustment [CR, RR=4.31 (0.36, 52.32); CR+Cri, RR=2.00 (0.46, 8.73)] and stepwise adjustment [CR, RR=3.41 (0.28, 41.94); CR+CRi, RR=1.97 (0.45, 8.65)].
CONCLUSIONS: In the absence of clinical trials directly testing less intensive AML treatments, indirect comparison methods apply statistically robust techniques versus naïve comparisons to measure comparative effectiveness. Regarding the likelihood of response to treatment, GLAS+LDAC showed improvement over AZA or DEC. As previously found when comparing OS, both ITC and STC estimated GLAS+LDAC superiority over AZA for both CR and CR+CRi. For GLAS+LDAC versus DEC, ITC and STC found numerical improvement in CR and CR+CRi; however, these treatments were not statistically significant for each of the two response outcomes.
Figure 1. ITC and STC results for GLAS+LDAC versus AZA or DEC
Forsythe:Novartis: Consultancy. Bell:Pfizer: Employment, Equity Ownership. Cappelleri:Pfizer: Employment, Equity Ownership. Chan:Pfizer: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal